Researchers at the RIKEN Cluster for Pioneering Research in Japan have developed a promising method to deliver a drug to cancer cells without affecting surrounding tissues. Published in Nature Catalysis, The new technique involves the clever combination of an artificial “metalloenzyme” with a surface sugar chain that guides it to the desired cells.
In the field of organic synthetic chemistry, many metal catalysts have been developed with the capacity to help synthesize molecules such as drugs and other functional materials. Recently, researchers have begun to focus on chemical reactions in living bodies catalyzed by transition metals—elements belonging to groups 3 through 11 on the periodic table. However, they have run into difficulties: transition-metal catalysts are easily “quenched”, meaning they are inactivated by substances such as antioxidants, which are common in living organisms. Therefore, practical application of transition-metal catalysts in living material has been a difficult challenge to overcome.
The international research team including Chief Scientist Katsunori Tanaka of RIKEN CPR and Special Postdoctoral Researcher Kenward Vong from the RIKEN Baton Zone Program developed an artificial “metalloenzyme” that contains a metal ion within a protective protein. The protein prevents the metal ion from being quenched, making it possible for the chemical reaction to take place in vivo. The metal ion in this case was ruthenium, which catalyzes an inactive precursor molecule into umbelliprenin, a plant-derived compound known to act against cancer cells. By attaching a sugar-chain “delivery tag” to the surface of the artificial metalloenzyme, they were then able to target it specifically to cancer cells where the drug was needed.
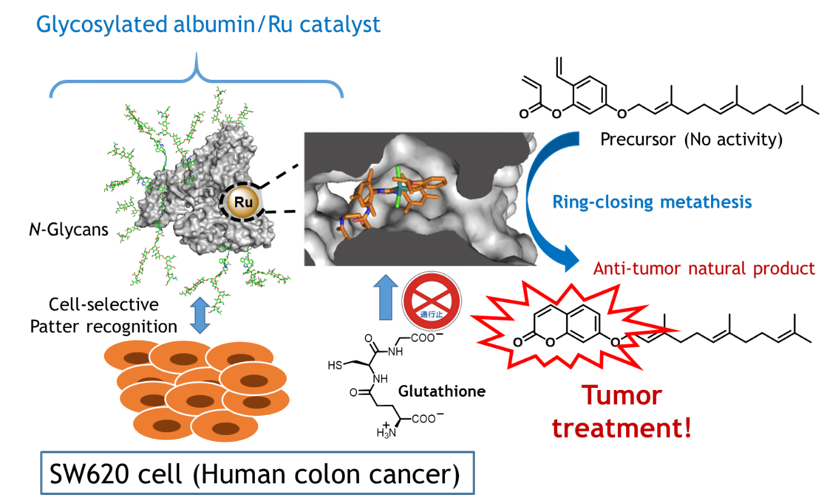
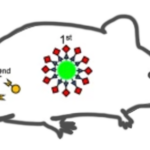
A newly developed technique delivers a drug to cancer cells by using an artificial metalloenzyme. The protien protects a metal catalyst (Ru) that acts to synthesize the drug once the metalloenzyme reaches the cancer cells. A specific sugar chain (N-Glycans) guides the metalloenzyme to the target cells.
To make the artificial enzyme, the group worked with a protein called human serum albumin, which is abundant in the human body. The researchers introduced a ruthenium catalyst into the hydrophobic pocket inside the protein. They found that in vitro, the ruthenium was able to carry out chemical reactions. “We were pleasantly surprised,” says Tanaka, who led the group, “that our newly developed metalloenzyme worked well in the presence of glutathione, an antioxidant that is abundant in actual cells and can inactivate ruthenium. With these results, we knew that the ruthenium catalyst was well protected from hydrophilic components such as glutathione, and at the same time could catalyze hydrophobic compounds that could enter the pocket.”
After determining that the catalysis would work, the researchers modified the surface of the albumin by attaching specific sugar chains that allowed the metalloenzyme to bind only to the target cancer cells. Once successfully delivered to cancer cells, the metal catalyst within the artificial enzyme could safely produce umbelliprenin, which they determined actually had cytotoxic effects on the cancer cells.
“We also confirmed that the method we developed can be applied to metal-catalyzed reactions using other catalysts such as gold, and that artificial metalloenzymes could be generally used in vivo,” adds Tanaka. “If transition-metal catalysis can be performed on specific organs or diseased cells in the body, it will allow us to rapidly and stably synthesize drugs at the site of the problem, thus minimizing side effects. Our findings could become a key in the fight against such diseases. Furthermore, we can consider using other natural compounds, which show strong anti-cancer activity but have yet to be used in treatments. We have opened a door to a new era in which we can synthesize and activate natural chemical compounds in actual organisms.” ✅
In the field of organic synthetic chemistry, many metal catalysts have been developed with the capacity to help synthesize molecules such as drugs and other functional materials. Recently, researchers have begun to focus on chemical reactions in living bodies catalyzed by transition metals—elements belonging to groups 3 through 11 on the periodic table. However, they have run into difficulties: transition-metal catalysts are easily “quenched”, meaning they are inactivated by substances such as antioxidants, which are common in living organisms. Therefore, practical application of transition-metal catalysts in living material has been a difficult challenge to overcome.
The international research team including Chief Scientist Katsunori Tanaka of RIKEN CPR and Special Postdoctoral Researcher Kenward Vong from the RIKEN Baton Zone Program developed an artificial “metalloenzyme” that contains a metal ion within a protective protein. The protein prevents the metal ion from being quenched, making it possible for the chemical reaction to take place in vivo. The metal ion in this case was ruthenium, which catalyzes an inactive precursor molecule into umbelliprenin, a plant-derived compound known to act against cancer cells. By attaching a sugar-chain “delivery tag” to the surface of the artificial metalloenzyme, they were then able to target it specifically to cancer cells where the drug was needed.
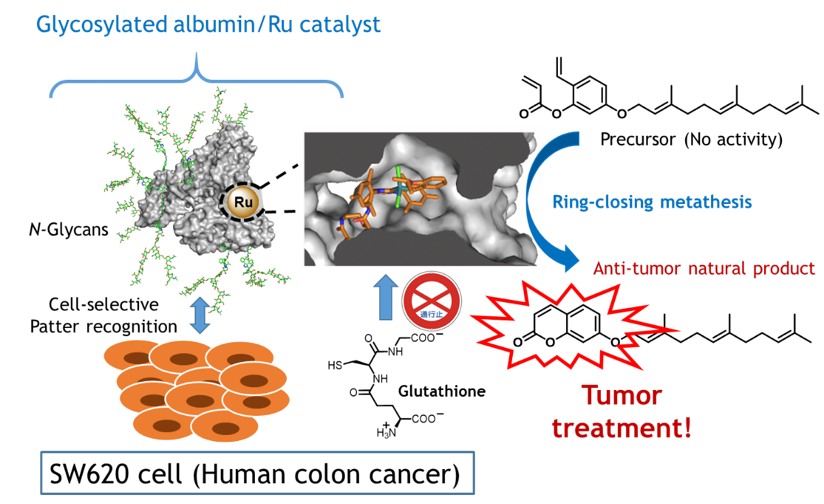
A newly developed technique delivers a drug to cancer cells by using an artificial metalloenzyme. The protien protects a metal catalyst (Ru) that acts to synthesize the drug once the metalloenzyme reaches the cancer cells. A specific sugar chain (N-Glycans) guides the metalloenzyme to the target cells.
To make the artificial enzyme, the group worked with a protein called human serum albumin, which is abundant in the human body. The researchers introduced a ruthenium catalyst into the hydrophobic pocket inside the protein. They found that in vitro, the ruthenium was able to carry out chemical reactions. “We were pleasantly surprised,” says Tanaka, who led the group, “that our newly developed metalloenzyme worked well in the presence of glutathione, an antioxidant that is abundant in actual cells and can inactivate ruthenium. With these results, we knew that the ruthenium catalyst was well protected from hydrophilic components such as glutathione, and at the same time could catalyze hydrophobic compounds that could enter the pocket.”
After determining that the catalysis would work, the researchers modified the surface of the albumin by attaching specific sugar chains that allowed the metalloenzyme to bind only to the target cancer cells. Once successfully delivered to cancer cells, the metal catalyst within the artificial enzyme could safely produce umbelliprenin, which they determined actually had cytotoxic effects on the cancer cells.
“We also confirmed that the method we developed can be applied to metal-catalyzed reactions using other catalysts such as gold, and that artificial metalloenzymes could be generally used in vivo,” adds Tanaka. “If transition-metal catalysis can be performed on specific organs or diseased cells in the body, it will allow us to rapidly and stably synthesize drugs at the site of the problem, thus minimizing side effects. Our findings could become a key in the fight against such diseases. Furthermore, we can consider using other natural compounds, which show strong anti-cancer activity but have yet to be used in treatments. We have opened a door to a new era in which we can synthesize and activate natural chemical compounds in actual organisms.” ✅
Further reading
Eda et al. (2019) Biocompatibility and therapeutic potential of glycosylated albumin artificial metalloenzymes. Nature Catalysis. doi: 10.1038/s41929-019-0317-4.