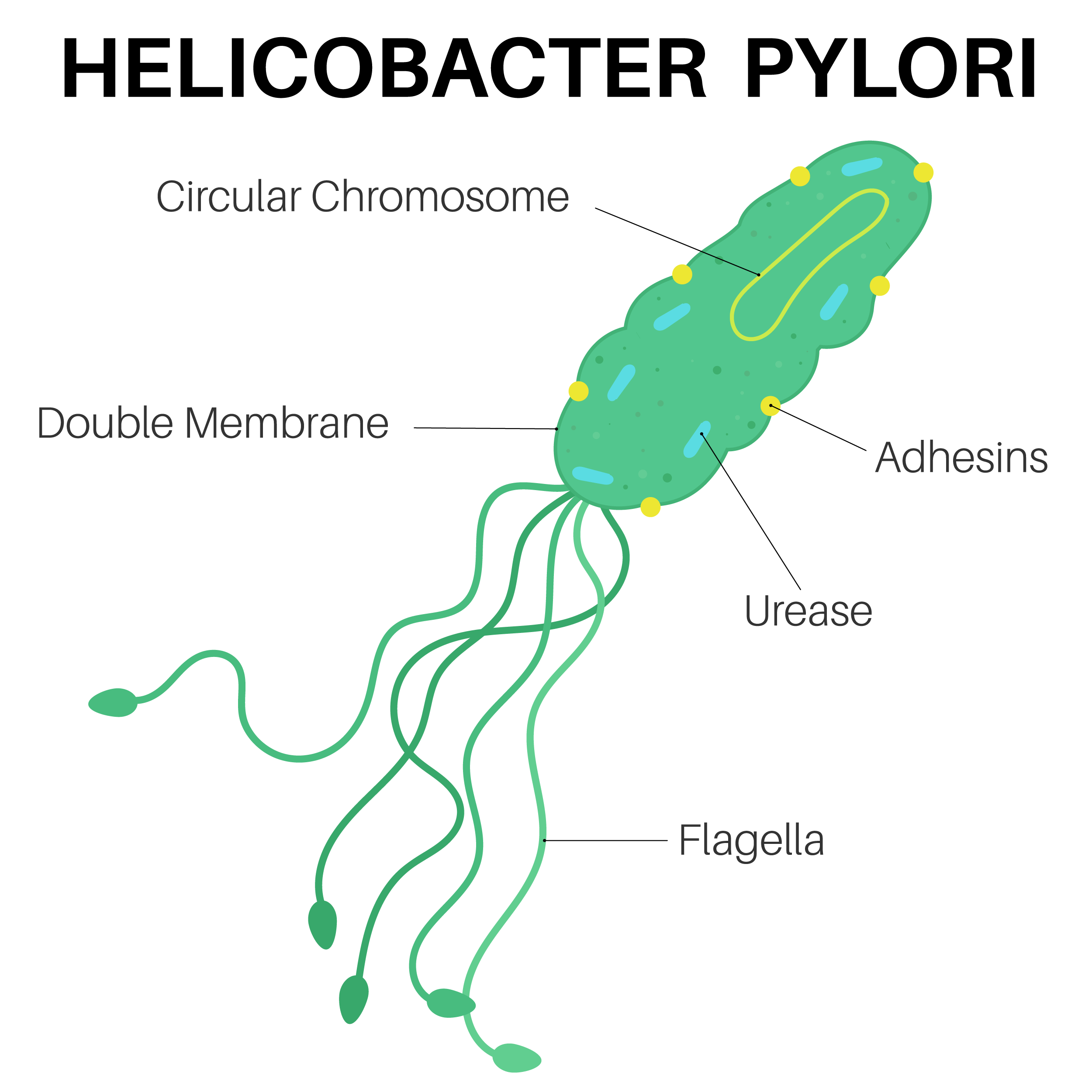
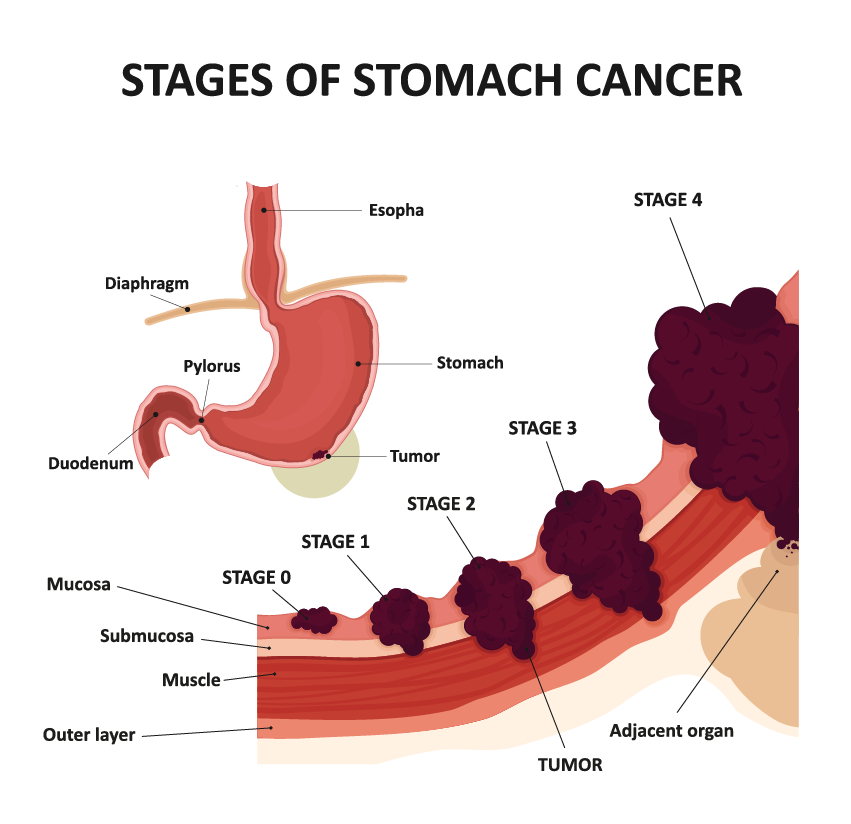
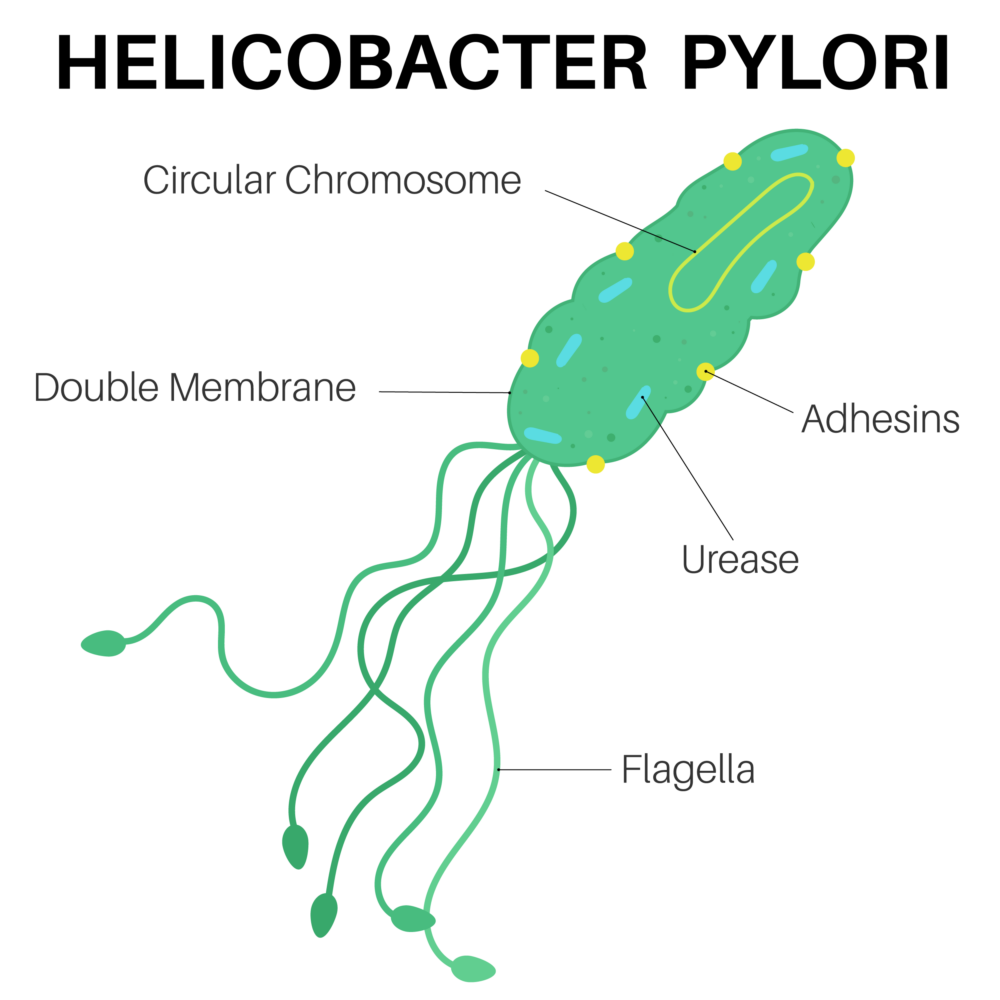
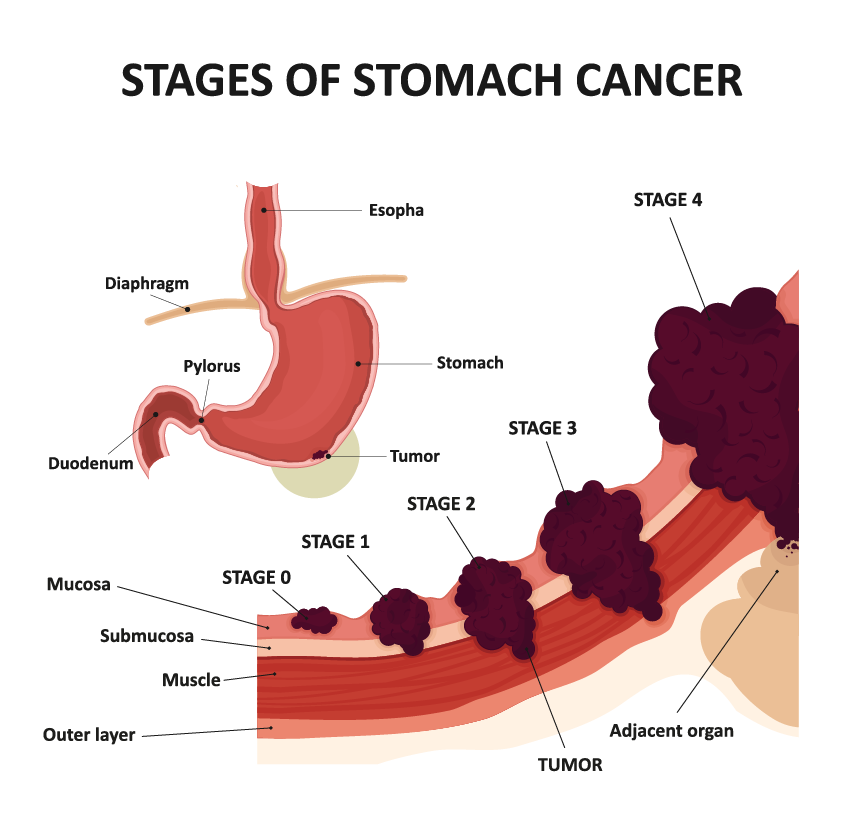
Alarge case-control study by international researchers at the RIKEN Center for Integrative Medical Sciences (IMS) in Japan has found that people who carry certain genetic risk factors for gastric (stomach) cancer have a much greater risk if they have also been infected by the bacterium Helicobacter pylori. The study, published in the medical journal The New England Journal of Medicine, could contribute to the development of tailored genomic medicine for treating stomach cancer.
Stomach cancer is the fourth leading cause of cancer death worldwide and has both environmental and genetic risk factors. Environmentally, infection by H. pylori increases the risk of stomach cancer. Because the virulence of H. pylori in East Asia is high, the incidence of stomach cancer is higher in countries like Japan. Genetically, while hereditary gene variation is why we have different colored eyes and are unique as individuals, sometimes gene variants are associated with the risk of disease. For example, individuals who carry a certain hereditary pathogenic variant of the CDH1 gene have an increased risk of gastric cancer.
Testing for the presence of pathogenic variants is now one of several measures being taken for cancer prevention, surveillance, and treatment selection. However, because large-scale, case-control studies are lacking, and because those that exist have not assessed how the risk for stomach cancer changes when pathogenic variants interact with environmental factors like H. pylori, it remains unclear what actual clinical measures can be taken. To address this issue, researchers therefore evaluated the risk of gastric cancer in a large case-control study of Japanese people, considering whether they were carriers of pathogenic variants and whether they had been infected by H. pylori.
Using a method for genomic analysis originally developed at RIKEN, the joint team led by Yukihide Momozawa at RIKEN IMS and Keitaro Matsuo at Aichi Cancer Center analyzed DNA samples from more than 11,000 patients with stomach cancer and 44,000 people without cancer for 27 genes associated with hereditary tumors. Their analysis identified nine genes that were highly associated with the risk for stomach cancer.
Next, the researchers analyzed the interaction between the pathogenic variants in the nine genes and patient history of H. pylori infection. First, they found that the risk for gastric cancer was dramatically higher when a pathogenic variant was combined with H. pylori infection than when either factor was present alone. Among the nine genes, four were of particular interest because they code for proteins that normally help repair damaged DNA. First author Yoshiaki Usui thinks that these pathogenic variants exacerbate the damage done by H. pylori infection. “H. pylori infection leads to cancer because it promotes DNA double-strand breaks and destabilizes the DNA of stomach cells,” explains Usui. “Combining that with genetic variants that prevent normal damage repair appears to substantially enhance the risk for gastric cancer.”
As the prevalence of H. pylori infection is high, and its eradication has proven difficult, screening for pathogenic variants can help determine who should be prioritized for interventions. Overall, reducing the risk of stomach cancer by testing for H. pylori infection and eradicating it remains a high priority for everyone, regardless of whether they carry the pathogenic variants. “For clinical practice, we will have to determine the extent to which H. pylori eradication actually has a preventive effect, or when eradication should be considered,” says Matsuo.
This study is part of a broad effort to develop better ways to prevent and treat cancer by understanding how environmental and genetic factors interact. “The information obtained from our study will contribute to medical practice guidelines on gastric cancer and pathogenic variants, and is expected to contribute to establishing a tailor-made genomic medicine system, including improved diagnostic accuracy, development of therapies targeting causative genes, and more appropriate preventive measures for gastric cancer,” says Momozawa.
Stomach cancer is the fourth leading cause of cancer death worldwide and has both environmental and genetic risk factors. Environmentally, infection by H. pylori increases the risk of stomach cancer. Because the virulence of H. pylori in East Asia is high, the incidence of stomach cancer is higher in countries like Japan. Genetically, while hereditary gene variation is why we have different colored eyes and are unique as individuals, sometimes gene variants are associated with the risk of disease. For example, individuals who carry a certain hereditary pathogenic variant of the CDH1 gene have an increased risk of gastric cancer.
Testing for the presence of pathogenic variants is now one of several measures being taken for cancer prevention, surveillance, and treatment selection. However, because large-scale, case-control studies are lacking, and because those that exist have not assessed how the risk for stomach cancer changes when pathogenic variants interact with environmental factors like H. pylori, it remains unclear what actual clinical measures can be taken. To address this issue, researchers therefore evaluated the risk of gastric cancer in a large case-control study of Japanese people, considering whether they were carriers of pathogenic variants and whether they had been infected by H. pylori.
Using a method for genomic analysis originally developed at RIKEN, the joint team led by Yukihide Momozawa at RIKEN IMS and Keitaro Matsuo at Aichi Cancer Center analyzed DNA samples from more than 11,000 patients with stomach cancer and 44,000 people without cancer for 27 genes associated with hereditary tumors. Their analysis identified nine genes that were highly associated with the risk for stomach cancer.
Next, the researchers analyzed the interaction between the pathogenic variants in the nine genes and patient history of H. pylori infection. First, they found that the risk for gastric cancer was dramatically higher when a pathogenic variant was combined with H. pylori infection than when either factor was present alone. Among the nine genes, four were of particular interest because they code for proteins that normally help repair damaged DNA. First author Yoshiaki Usui thinks that these pathogenic variants exacerbate the damage done by H. pylori infection. “H. pylori infection leads to cancer because it promotes DNA double-strand breaks and destabilizes the DNA of stomach cells,” explains Usui. “Combining that with genetic variants that prevent normal damage repair appears to substantially enhance the risk for gastric cancer.”
As the prevalence of H. pylori infection is high, and its eradication has proven difficult, screening for pathogenic variants can help determine who should be prioritized for interventions. Overall, reducing the risk of stomach cancer by testing for H. pylori infection and eradicating it remains a high priority for everyone, regardless of whether they carry the pathogenic variants. “For clinical practice, we will have to determine the extent to which H. pylori eradication actually has a preventive effect, or when eradication should be considered,” says Matsuo.
This study is part of a broad effort to develop better ways to prevent and treat cancer by understanding how environmental and genetic factors interact. “The information obtained from our study will contribute to medical practice guidelines on gastric cancer and pathogenic variants, and is expected to contribute to establishing a tailor-made genomic medicine system, including improved diagnostic accuracy, development of therapies targeting causative genes, and more appropriate preventive measures for gastric cancer,” says Momozawa.
Further reading
Usui et al. (2023) Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N Engl J Med. doi:10.1056/NEJMoa2211807
Further reading
Usui et al. (2023) Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N Engl J Med. doi:10.1056/NEJMoa2211807