Arecent study led by Hidenori Machino at the RIKEN Center for Advanced Intelligence Project (AIP) and the National Cancer Center Research Institute in Japan used a big data multi-omics analysis to examine changes in gene expression as cells from human fallopian tubes become cancerous. After identifying dysregulation in several biological signaling pathways, they were able to both predict an effective treatment and test it, with promising results. The study was published in the scientific journal Experimental & Molecular Medicine.
Ovarian cancer is one the most challenging cancers affecting the female reproductive system, with high-grade serous ovarian carcinoma (HGSOC) being the deadliest. This type of cancer, like many others, is not driven by a single mutation, which makes it more difficult to treat. Because of this, rather than looking at DNA sequences, the team focused on epigenetic profiles—the on/off switches within a specific cell type that affect gene expression and, in this case, lead to tumor formation.
HGSOC originates in the fallopian tubes, with the most difficult cases being unresponsive to chemotherapy. The researchers zeroed in on this type of tumor using cells derived from human fallopian tube epithelial cells, which they grew in various conditions and studied using a special integrative omics analysis. “This analysis integrates and analyzes a huge amount of data from multiple high-throughput techniques, including ATAC, ChIP, and RNA sequencing, to gain a holistic understanding of complex biological systems,” says Machino.
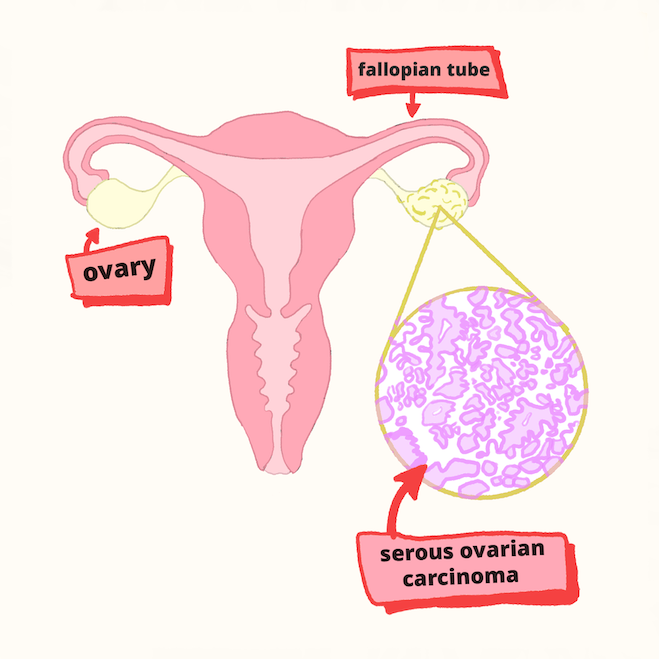
Multi-omics analysis predicted that specific factors which control gene expression behave abnormally during ovarian tumorigenesis. This led to a potential treatment for high-grade serous ovarian carcinoma, one of the most deadly cancers that does not respond well to chemotherapy.
The multi-omics analysis predicted that specific factors which control gene expression behave abnormally during tumorigenesis, just when cells transition from being normal to being cancerous. These predictions were tested by comparing protein levels between normal and cancerous cells. The predictions held, and the researchers found that certain proteins, known as the AP-1 complex, were overly active in cancerous cells. These proteins play a role in spurring the growth and spread of cancer cells. Additionally, another set of proteins, the GATA family, which usually helps control cell behavior, was found to be less effective in cancerous cells.
The analysis also identified specific genes –MAF, GATA6, and DAB2 – that play a crucial role in controlling cancer growth. In early tumorigenesis, these genes were epigenetically suppressed, contributing to tumor formation. By understanding how suppressing these genes led to dysfunction, the researchers were able to deduce a countermeasure. “We realized that the culprit was excessive Ras activation as a result of the epigenetic gene suppression,” says Machino, “and reasoned that a drug which can block events in this pathway would reverse the trend.” When tested with trametinib, a clinically applicable drug that can inhibit Ras signaling, they observed signs of normal epigenetic control, including de-suppression of MAF and DAB2.
Drugs like trametinib are called MEK inhibitors and this study predicts that they could be effective in preventing tumorigenesis in ovarian cancer. In addition, suppressed MAF, GATA6, and DAB2 could be useful biomarkers. “The HGSOC biomarkers we discovered have the potential to be used for early detection of ovarian cancer,” says Machino. “The findings also point to new therapeutic approaches, which could have a significant impact on society.”
Ovarian cancer is one the most challenging cancers affecting the female reproductive system, with high-grade serous ovarian carcinoma (HGSOC) being the deadliest. This type of cancer, like many others, is not driven by a single mutation, which makes it more difficult to treat. Because of this, rather than looking at DNA sequences, the team focused on epigenetic profiles—the on/off switches within a specific cell type that affect gene expression and, in this case, lead to tumor formation.
HGSOC originates in the fallopian tubes, with the most difficult cases being unresponsive to chemotherapy. The researchers zeroed in on this type of tumor using cells derived from human fallopian tube epithelial cells, which they grew in various conditions and studied using a special integrative omics analysis. “This analysis integrates and analyzes a huge amount of data from multiple high-throughput techniques, including ATAC, ChIP, and RNA sequencing, to gain a holistic understanding of complex biological systems,” says Machino.
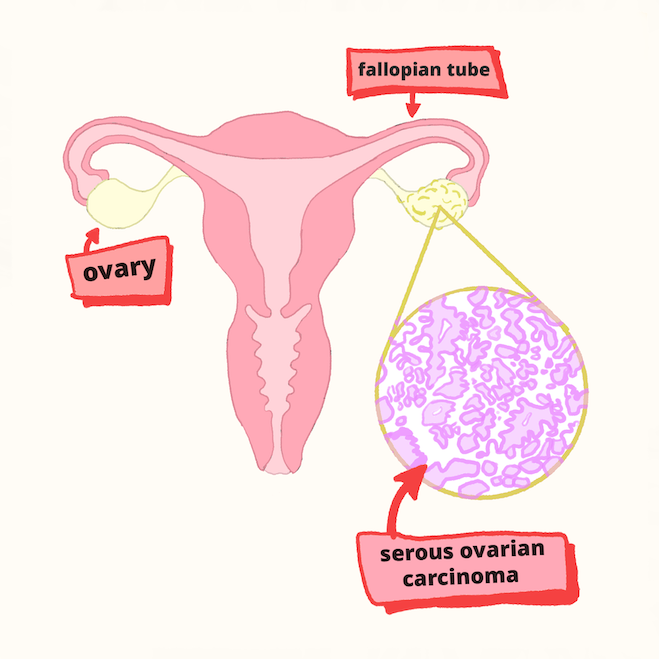
Multi-omics analysis predicted that specific factors which control gene expression behave abnormally during ovarian tumorigenesis. This led to a potential treatment for high-grade serous ovarian carcinoma, one of the most deadly cancers that does not respond well to chemotherapy.
The multi-omics analysis predicted that specific factors which control gene expression behave abnormally during tumorigenesis, just when cells transition from being normal to being cancerous. These predictions were tested by comparing protein levels between normal and cancerous cells. The predictions held, and the researchers found that certain proteins, known as the AP-1 complex, were overly active in cancerous cells. These proteins play a role in spurring the growth and spread of cancer cells. Additionally, another set of proteins, the GATA family, which usually helps control cell behavior, was found to be less effective in cancerous cells.
The analysis also identified specific genes –MAF, GATA6, and DAB2 – that play a crucial role in controlling cancer growth. In early tumorigenesis, these genes were epigenetically suppressed, contributing to tumor formation. By understanding how suppressing these genes led to dysfunction, the researchers were able to deduce a countermeasure. “We realized that the culprit was excessive Ras activation as a result of the epigenetic gene suppression,” says Machino, “and reasoned that a drug which can block events in this pathway would reverse the trend.” When tested with trametinib, a clinically applicable drug that can inhibit Ras signaling, they observed signs of normal epigenetic control, including de-suppression of MAF and DAB2.
Drugs like trametinib are called MEK inhibitors and this study predicts that they could be effective in preventing tumorigenesis in ovarian cancer. In addition, suppressed MAF, GATA6, and DAB2 could be useful biomarkers. “The HGSOC biomarkers we discovered have the potential to be used for early detection of ovarian cancer,” says Machino. “The findings also point to new therapeutic approaches, which could have a significant impact on society.”
Further reading
Machino et al. (2023) Integrative analysis reveals early epigenetic alterations in high-grade serous ovarian carcinomas. Exp Mol Med. doi:10.1038/s12276-023-01090-1
Further reading
Machino et al. (2023) Integrative analysis reveals early epigenetic alterations in high-grade serous ovarian carcinomas. Exp Mol Med. doi:10.1038/s12276-023-01090-1










