Researchers led by Hiroshi Ohno at the RIKEN Center for Integrative Medical Sciences (IMS) in Japan have discovered a type of gut bacteria that might help improve insulin resistance, and thus protect against the development of obesity and type-2 diabetes. The study, published August 30 in the scientific journal Nature, involved genetic and metabolic analysis of human fecal microbiomes and then corroborating experiments in obese mice.
Insulin is a hormone released by the pancreas in response to blood sugar. Normally, it helps get the sugar into the muscles and liver so that they can use the energy. When someone develops insulin resistance, it means that insulin is prevented from doing its job, and as a result, more sugar stays in their blood and their pancreas continues to make more insulin. Insulin resistance can lead to obesity, pre-diabetes, and full-blown type-2 diabetes.
The food we eat can determine which bacteria dominate our guts. Now we know that in addition to our mood and immune system, some gut bacteria can influence insulin resistance, both negatively and positively.
Our guts contain trillions of bacteria, many of which break down the carbohydrates that we eat when they would otherwise remain undigested. While many have proposed that this phenomenon is related to obesity and pre-diabetes, the facts remain unclear because there are so many different bacteria and there is a lack of metabolic data. Ohno and his team at RIKEN IMS have addressed this lack with their comprehensive study, and in the process, discovered a type of bacteria that might help reduce insulin resistance.
First, they examined as many metabolites as they could detect in the feces provided by over 300 adults at their regular health checkups. They compared this metabolome with the insulin resistance levels obtained from the same people. “We found that higher insulin resistance was associated with excessive carbohydrates in the fecal matter,” says Ohno, “especially monosaccharides like glucose, fructose, galactose, and mannose.”
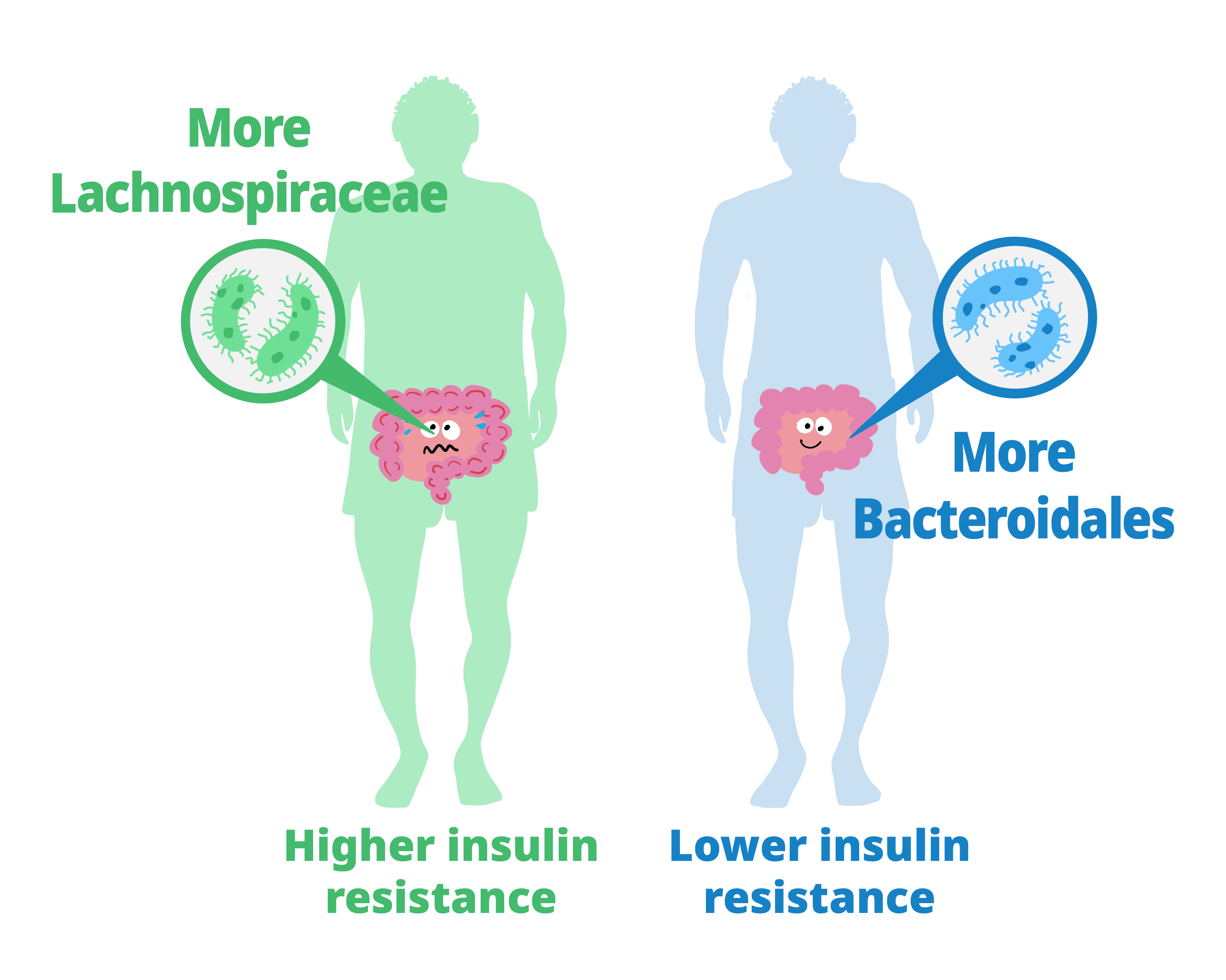
The study showed that people whose gut bacteria are dominated by Lachnospiraceae tend to have higher levels of insulin resistance and higher fecal monosaccharide content. Those with more Bacteroidales tended to have lower insulin resistance and lower fecal monosaccharide content.
Next, they characterized the gut microbiota of the study participants and their relationship with insulin resistance and fecal carbohydrates. The guts of people with higher insulin resistance contained more bacteria from the taxonomic order c than from other orders. Additionally, microbiomes that included Lachnospiraceae were associated with excess fecal carbohydrates. Thus, a gut microbiota dominated by Lachnospiraceae was related to both insulin resistance and feces with excessive monosaccharides. At the same time, insulin resistance and monosaccharide levels were lower in participants whose guts contained more Bacteroidales-type bacteria than other types.
The team then set out to see the direct effect of bacteria on metabolism in culture and then in mice. In culture, Bacteroidales bacteria consumed the same kinds of monosaccharides that were found in the feces of people with high insulin resistance, with the species Alistipes indistinctus consuming the greatest variety. In obese mice, the team looked at how treatment with different bacteria affected blood sugar levels. They found that A. indistinctus lowered blood sugar and reduced insulin resistance and the amount of carbohydrates available to the mice.
These results were compatible with the findings from human patients and have implications for diagnosis and treatment. As Ohno explains, “Because of its association with insulin resistance, the presence of gut Lachnospiraceae bacteria could be a good biomarker for pre-diabetes. Likewise, treatment with probiotics containing A. indistinctus might improve glucose intolerance in those with pre-diabetes.”
Although most over-the-counter probiotics do not currently contain the bacteria identified in this study, Ohno urges caution should they become available. “These findings need to be verified in human clinical trials before we can recommend any probiotic as treatment for insulin resistance.”
Insulin is a hormone released by the pancreas in response to blood sugar. Normally, it helps get the sugar into the muscles and liver so that they can use the energy. When someone develops insulin resistance, it means that insulin is prevented from doing its job, and as a result, more sugar stays in their blood and their pancreas continues to make more insulin. Insulin resistance can lead to obesity, pre-diabetes, and full-blown type-2 diabetes.
The food we eat can determine which bacteria dominate our guts. Now we know that in addition to our mood and immune system, some gut bacteria can influence insulin resistance, both negatively and positively.
Our guts contain trillions of bacteria, many of which break down the carbohydrates that we eat when they would otherwise remain undigested. While many have proposed that this phenomenon is related to obesity and pre-diabetes, the facts remain unclear because there are so many different bacteria and there is a lack of metabolic data. Ohno and his team at RIKEN IMS have addressed this lack with their comprehensive study, and in the process, discovered a type of bacteria that might help reduce insulin resistance.
First, they examined as many metabolites as they could detect in the feces provided by over 300 adults at their regular health checkups. They compared this metabolome with the insulin resistance levels obtained from the same people. “We found that higher insulin resistance was associated with excessive carbohydrates in the fecal matter,” says Ohno, “especially monosaccharides like glucose, fructose, galactose, and mannose.”
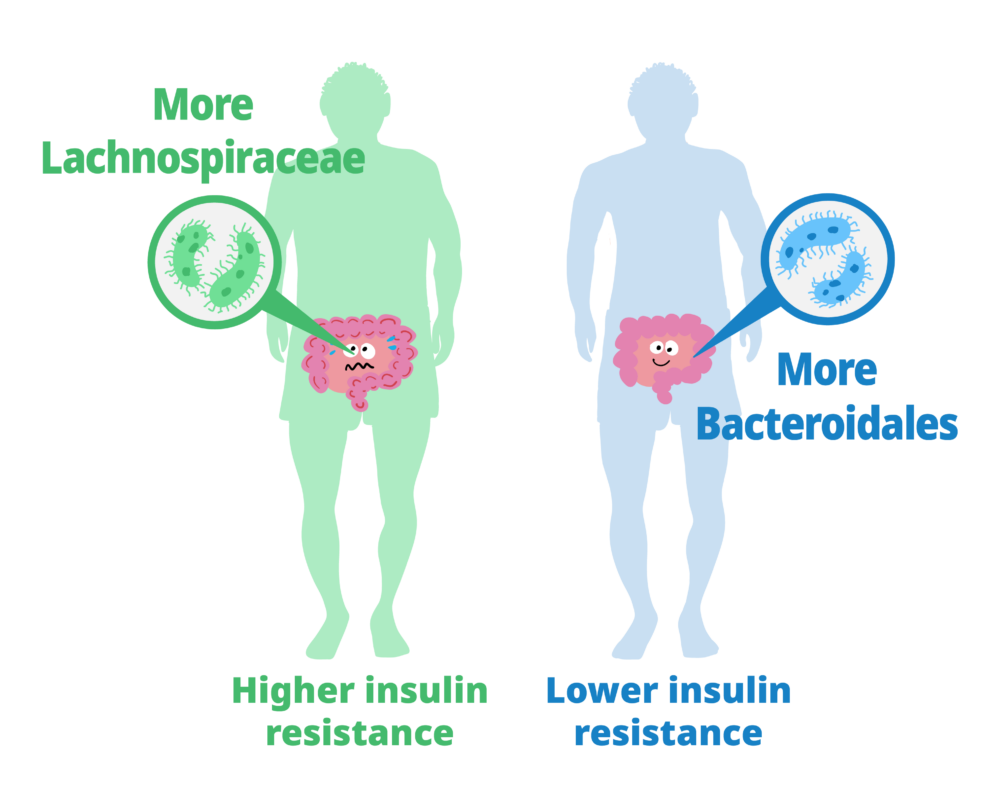
The study showed that people whose gut bacteria are dominated by Lachnospiraceae tend to have higher levels of insulin resistance and higher fecal monosaccharide content. Those with more Bacteroidales tended to have lower insulin resistance and lower fecal monosaccharide content.
Next, they characterized the gut microbiota of the study participants and their relationship with insulin resistance and fecal carbohydrates. The guts of people with higher insulin resistance contained more bacteria from the taxonomic order c than from other orders. Additionally, microbiomes that included Lachnospiraceae were associated with excess fecal carbohydrates. Thus, a gut microbiota dominated by Lachnospiraceae was related to both insulin resistance and feces with excessive monosaccharides. At the same time, insulin resistance and monosaccharide levels were lower in participants whose guts contained more Bacteroidales-type bacteria than other types.
The team then set out to see the direct effect of bacteria on metabolism in culture and then in mice. In culture, Bacteroidales bacteria consumed the same kinds of monosaccharides that were found in the feces of people with high insulin resistance, with the species Alistipes indistinctus consuming the greatest variety. In obese mice, the team looked at how treatment with different bacteria affected blood sugar levels. They found that A. indistinctus lowered blood sugar and reduced insulin resistance and the amount of carbohydrates available to the mice.
These results were compatible with the findings from human patients and have implications for diagnosis and treatment. As Ohno explains, “Because of its association with insulin resistance, the presence of gut Lachnospiraceae bacteria could be a good biomarker for pre-diabetes. Likewise, treatment with probiotics containing A. indistinctus might improve glucose intolerance in those with pre-diabetes.”
Although most over-the-counter probiotics do not currently contain the bacteria identified in this study, Ohno urges caution should they become available. “These findings need to be verified in human clinical trials before we can recommend any probiotic as treatment for insulin resistance.”
Further reading
Takeuchi, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature (2023). doi: 10.1038/s41586-023-06466-x
Further reading
Takeuchi, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature (2023). doi: 10.1038/s41586-023-06466-x