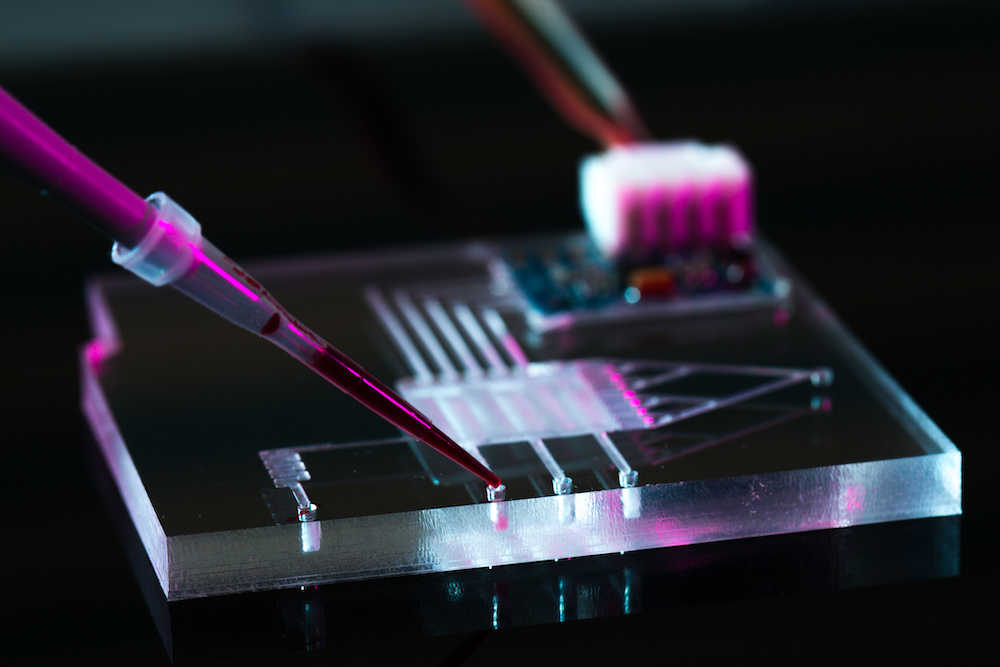
Researchers at the RIKEN Center for Biosystems Dynamics Research in Japan have developed a new system for keeping tissue viable for long-term study once transferred from an animal to a culture medium. The new system uses a microfluidic device that can keep tissue from both drying out and from drowning in fluid. A proof-of-concept experiment showed that tissue explanted from the mouse brain remained viable after almost one month in culture, much longer than is possible with other microfluidic culturing methods, and also much simpler.
Experimenting on tissues in culture can facilitate drug discovery because researchers can systematically manipulate the tissue and test different drugs or drug combinations. However, when studying a whole system in which many cells must interact with each other, it has proven difficult to keep the tissue “alive” for more than a few days. Tissue dries out quickly and dies unless it is put into a wet culture medium with appropriate nutrients. On the other hand, immersing complex tissue in fluid can damage the tissue because it does not allow the normal transfer of gases between them.
To solve this problem, the RIKEN scientists developed a microfluidic device using polydimethylsiloxane (PDMS), the material often used as a defoamer in over-the-counter drugs. The device has a semi-permeable channel surrounded by an artificial membrane and solid PDMS walls. Rather than constantly being immersed in fluid, the tissue benefited from having the culture medium circulate within the microchannel and pass through the permeable membrane, which allowed proper gas exchange. This sounds simple, but finding the optimal settings proved challenging. As first author Nobutoshi Ota notes, “Controlling the medium flow was difficult because the microchannel that formed between the PDMS walls and the porous membrane was unusual. However, we had success after trial and error modifications to the porous membrane and adjustments of the inlet/outlet flow rates.”
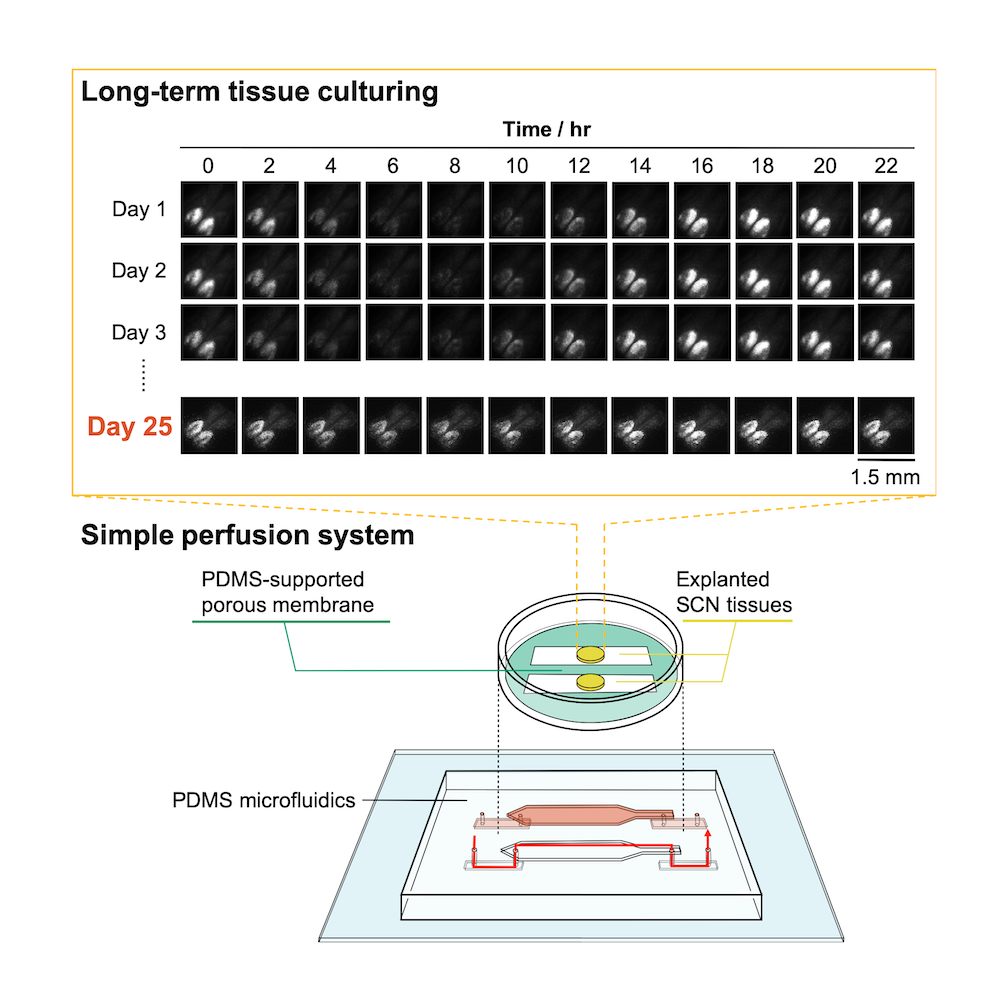
Bottom: the microfluidic culture device setup with explanted suprachiasmatic nucleus (SCN) tissue. Top: rhythmic circadian activity was still evident after 25 days, as indicated by bioluminescence imaging.
The team tested the device using tissue from the mouse suprachiasmatic nucleus, a complex part of the brain that governs circadian rhythms. The mice themselves were knock-in mice in which circadian rhythm activity in the brain was linked to the production of a highly fluorescent protein. By measuring the level of bioluminescence coming from the brain tissue, they were able to see that tissue kept alive by their system stayed active and functional for over 25 days with nice circadian activity. In contrast, neural activity in tissue kept in a conventional culture decreased by 6% after only 10 hours.
This new method will have several benefits. In the short-term, it will be useful in observing biological development and testing how tissues respond to drugs. The long-term benefits are also clear. “This method can be used for more than explanted tissues from animals,” says Ota. “It will also improve research into organogenesis through long-term culturing and observation which is necessary for growing tissue and organs.”
Indeed, the team is currently planning long-term experiments using their system to observe the formation of blood vessels and the movements of cells during organoid formation.
This study was published in the journal Analytical Sciences. ✅
Experimenting on tissues in culture can facilitate drug discovery because researchers can systematically manipulate the tissue and test different drugs or drug combinations. However, when studying a whole system in which many cells must interact with each other, it has proven difficult to keep the tissue “alive” for more than a few days. Tissue dries out quickly and dies unless it is put into a wet culture medium with appropriate nutrients. On the other hand, immersing complex tissue in fluid can damage the tissue because it does not allow the normal transfer of gases between them.
To solve this problem, the RIKEN scientists developed a microfluidic device using polydimethylsiloxane (PDMS), the material often used as a defoamer in over-the-counter drugs. The device has a semi-permeable channel surrounded by an artificial membrane and solid PDMS walls. Rather than constantly being immersed in fluid, the tissue benefited from having the culture medium circulate within the microchannel and pass through the permeable membrane, which allowed proper gas exchange. This sounds simple, but finding the optimal settings proved challenging. As first author Nobutoshi Ota notes, “Controlling the medium flow was difficult because the microchannel that formed between the PDMS walls and the porous membrane was unusual. However, we had success after trial and error modifications to the porous membrane and adjustments of the inlet/outlet flow rates.”
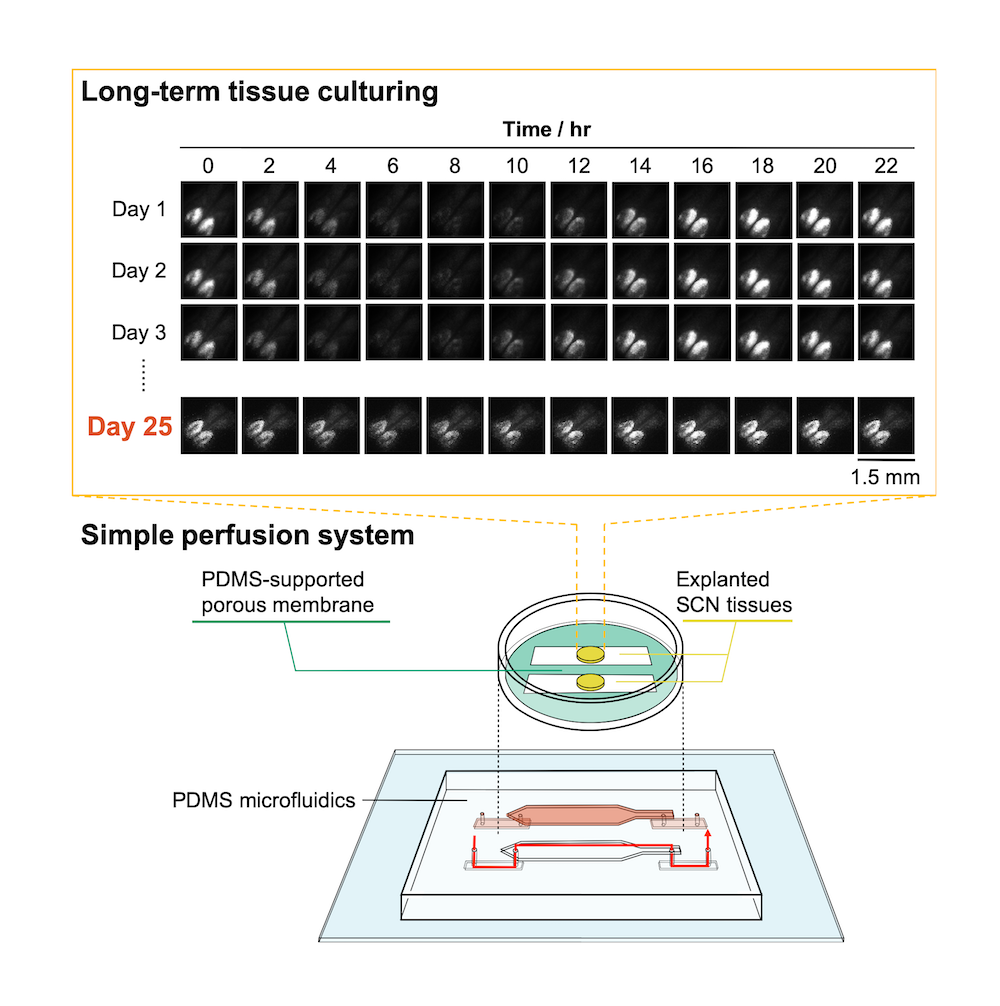
Bottom: the microfluidic culture device setup with explanted suprachiasmatic nucleus (SCN) tissue. Top: rhythmic circadian activity was still evident after 25 days, as indicated by bioluminescence imaging.
The team tested the device using tissue from the mouse suprachiasmatic nucleus, a complex part of the brain that governs circadian rhythms. The mice themselves were knock-in mice in which circadian rhythm activity in the brain was linked to the production of a highly fluorescent protein. By measuring the level of bioluminescence coming from the brain tissue, they were able to see that tissue kept alive by their system stayed active and functional for over 25 days with nice circadian activity. In contrast, neural activity in tissue kept in a conventional culture decreased by 6% after only 10 hours.
This new method will have several benefits. In the short-term, it will be useful in observing biological development and testing how tissues respond to drugs. The long-term benefits are also clear. “This method can be used for more than explanted tissues from animals,” says Ota. “It will also improve research into organogenesis through long-term culturing and observation which is necessary for growing tissue and organs.”
Indeed, the team is currently planning long-term experiments using their system to observe the formation of blood vessels and the movements of cells during organoid formation.
This study was published in the journal Analytical Sciences. ✅
Further reading
Ota et al. (2019) A microfluidic platform based on robust gas and liquid exchange for long-term culturing of explanted tissues. Anal Sci. doi: 10.2116/analsci.19p099.